Chemistry is the scientific study of matter, including its structure, properties, and the changes it undergoes. At the foundation of chemistry is the concept of atoms and elements. These fundamental units play a crucial role in the chemistry of life, particularly in understanding the composition and function of the human body. This note explores the basic structure of atoms, the distinction between atoms and elements, the major elements in the human body, and the role of isotopes and radioisotopes in chemistry.
Atoms and atomic structure
Atoms are the smallest units of matter that retain the chemical properties of an element. They are made up of three main subatomic particles: protons, neutrons and electrons.
- Protons They are positively charged particles found in the atomic nucleus. They have a mass of approximately 1 atomic mass unit (amu). The number of protons in an atom defines the element and is known as the atomic number. For example, all carbon atoms have 6 protons.
- Neutrons They are neutral particles (i.e. they have no charge) that also reside in the nucleus. They are slightly larger than protons and have a mass of about 1 amu. Neutrons contribute to the atomic mass, but do not affect the charge of the atom.
- Electrons They are negatively charged particles with negligible mass compared to protons and neutrons. They orbit the nucleus in regions called electron shells. Despite their minimal mass, electrons are crucial in chemical bonds and reactions.
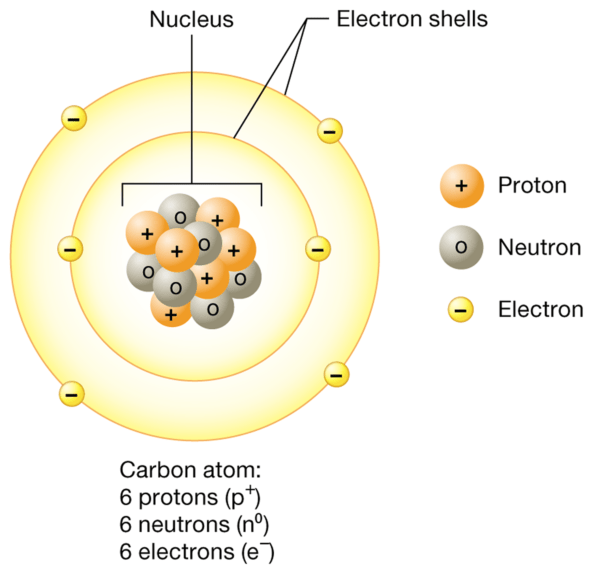
The nucleus, which contains protons and neutrons, is incredibly dense compared to the relatively large electron shells. Although the electrons are spread out over a large volume, most of an atom's mass is concentrated in the nucleus.
Electron layers
The classical model of the atom shows electrons orbiting the nucleus in a manner similar to planets around the sun. However, this is a simplification. Electrons exist in probability regions known as electron shells. The first shell can hold up to 2 electrons, the second shell up to 8, and the third shell up to 18. Electrons fill the lower energy levels (or shells) first before occupying the higher ones. This arrangement influences the way atoms interact chemically.
Atoms vs. elements
The terms “atom” and “element” are often used interchangeably but have different meanings:
- AtomAn atom is the smallest unit of an element that retains its chemical properties. It is made up of protons, neutrons, and electrons. Atoms can combine to form molecules, which are the basic components of matter.
- ElementAn element is a pure substance consisting of only one type of atom. Elements are defined by their atomic number, which is the number of protons in their atoms. For example, hydrogen (H) is an element whose atoms have one proton. There are currently 118 known elements, each with unique properties.
Main elements of the human body
The human body is mainly composed of a few key elements, which are essential for various biological functions:
- Oxygen (O)Oxygen, which makes up about 65% of the human body's mass, is crucial for cellular respiration, the process by which cells generate energy.
- Carbon (C)Carbon, which makes up about 18% of the body, is the backbone of organic molecules including carbohydrates, proteins, lipids, and nucleic acids.
- Hydrogen (H)Hydrogen, which makes up about 10% of the body, is a component of water and organic molecules and plays a vital role in maintaining pH balance and energy production.
- Nitrogen (N)Nitrogen, which makes up about 3% of the body, is a key element in amino acids, which are the building blocks of proteins, and in nucleic acids, which make up DNA and RNA.
Other elements, such as calcium, phosphorus, potassium, sulfur, sodium and magnesium, are present in smaller amounts but are still essential for maintaining health and supporting various physiological functions.
Atomic number, mass number, isotopes and radioisotopes
To understand the variation between atoms, we need to distinguish between atomic number, mass number, isotopes and radioisotopes:
- Atomic number:The atomic number is the number of protons in the nucleus of an atom. It defines the element and its position on the periodic table. For example, the atomic number of carbon is 6, which means that all carbon atoms have six protons.
- Mass number:The mass number is the sum of protons and neutrons in the nucleus of an atom. It represents the total nuclear mass of the atom. For example, carbon-12 has a mass number of 12, indicating a total of 6 protons and 6 neutrons.
- Isotopes:Isotopes are variants of the same element that have the same number of protons but different numbers of neutrons. This results in different mass numbers. For example, carbon has three isotopes: carbon-12 (6 protons and 6 neutrons), carbon-13 (6 protons and 7 neutrons), and carbon-14 (6 protons and 8 neutrons).
- Radioisotopes:Radioisotopes, or radioactive isotopes, are unstable isotopes that decay over time, emitting radiation in the process. This radioactive decay changes the isotope into a more stable form. Radioisotopes are used in a variety of applications, including medical diagnosis and treatment. For example, iodine-131 is used to treat thyroid disorders.
Production of isotopes
Isotopes are produced through several nuclear processes:
- Natural processes:Some isotopes occur naturally. For example, carbon-14 is continuously formed in the atmosphere through the interaction of cosmic rays with nitrogen-14.
- Artificial processes:Isotopes can also be created artificially in laboratories or nuclear reactors. By bombarding stable atoms with neutrons or other particles, scientists can produce isotopes with different mass numbers. For example, technetium-99m, used in medical imaging, is produced in nuclear reactors.
Conclusion
Understanding the basics of atomic structure, the distinction between atoms and elements, and the role of isotopes and radioisotopes is fundamental in chemistry and essential for exploring the chemistry of life. These concepts help elucidate the composition and function of biological molecules and the use of radioactive materials in medicine. By understanding these principles, we gain insight into how the smallest units of matter contribute to the complex processes that sustain life.